
Observational Study
 The Association of Hyperglycaemia with Complications in Patients with AcuteMyocardial Infarction-An Observational Study
The Association of Hyperglycaemia with Complications in Patients with AcuteMyocardial Infarction-An Observational Study
Abstract
Background: Acute myocardial infarction (AMI) is one of the most common causes of morbidity and mortalityglobally. It occurs when myocardial ischemia, the diminished blood supply to the heart, exceeds a particular thresholdand saturates the myocardial repair mechanisms capacity to maintain normal function and homeostasis. Furthermore,the presence of hyperglycaemia substantiates electrophysiological alteration, impairs left ventricular (LV) function andcauses perfusion defects promoting multiple cardiac complications viz. arrhythmias, cardiogenic shock, heart failureand death. Raised admission random blood sugar (RBS) signifies hyperglycaemia and HbA1c serves a prognosticpurpose in patients of myocardial infarction (MI) to be watchful regarding complications
Aim: The present study was carried out to study the association between admission blood glucose levels and thedevelopment of complications in patients having acute MI. We also studied the association of HbA1c levels withprognosis in MI patients.
Materials and Methods: 100 eligible consecutive patients of acute myocardial infarction (AMI) were enrolled andwere grouped according to their RBS whether <180 mg/mL (group 1) or >180 mg/mL (group 2) irrespective of priorhistory of diabetes. In a parallel scenario, these 100 patients were also evaluated for HbA1c levels and divided whether<6% (group 1) and >6% (group 2) and analysed using particle enhanced immune-turbidimetric test. Simultaneously,routine investigations viz., complete blood count, renal and liver profile, 2D echo and serial electrocardiogram (ECG)were performed.
Results: Biophysiological processes involving renin-angiotensin-aldosterone system (RAAS) activation, sympatheticmodalities and inflammatory cascade due to AMI induced stress potentiates reactionary hyperglycaemia whichevidently showed more risk of complications, thereby developing heart failure in 36% cases (group 2) contrasting 6%(group 1). Further, arrhythmias occurred in 30% (group 2) contrasting 6% (group 1) and shock in 26% (group 2) with6% (group 1). Hyperglycaemia was associated with higher mortality viz., 28% (group 2) in comparison to 6% (group1) with a significant difference. The HbA1c levels underlined higher complications viz., 23.1% (group 2) than 12.5%(group 1) with overall mortality higher in diabetics (group 2) in comparison with non-diabetics.
Conclusion: The study tends to highlight the stress response initiated by a myocardial injury that triggersproinflammatory cascades and sympathetic nervous system; causing catecholamine secretions and pseudo-emergencystates thus causing extensive myocardial damage and further deterioration in prognosis.
Keywords
Hyperglycaemia, myocardial infarction, cardiac arrhythmias, blood sugar, heart failure
Introduction
Ischaemic heart disease (IHD) causes more mortality and greater economic costs than any other illness in the developedworld. Cardiovascular diseases as of 2018 were the leading cause of death globally resulting in over 17.6 million deathsyearly worldwide in which IHD contributed 43.2% deaths and is expected to climb to more than 23.6 million by 2030.1 Acutemyocardial infarction, as a component of IHD, is a condition in which there are an inadequate blood and oxygen supply toa portion of the myocardium; typically presents when an imbalance occurs between myocardial oxygen supply and demand,most commonly due to atherosclerosis of an epicardial coronary artery (or arteries).2,3
The incidence of MI rises with atherosclerotic disease mainly predisposed by six primary risk factors viz. diabetesmellitus (DM), hypertension, tobacco use, dyslipidaemia, male sex and family history of arterial atherosclerosis.4 Occurrenceof myocardial ischaemia characterised mainly by cell death, takes variable duration depending upon the presence of collateralsto the ischaemic zone, persistent or intermittent coronary arterial occlusion, the myocytic sensitivity to ischaemia, preconditioning or finally, individual demand for myocardial oxygen and nutrients.
From a morphological viewpoint, the two types of MI are transmural and non-transmural, whereas on the basis ofelectrocardiographic findings are ST-elevation myocardial infarction (STEMI) or non-ST-elevation myocardial infarction(NSTEMI).5 Diagnosis is confirmed by ECG, serum cardiac biomarkers, cardiac imaging, and nonspecific indices of tissuenecrosis and inflammation. If untreated, the ischemia worsens manifesting as reduced ventricular compliance (diastolic failure)and/or a reduced stroke volume with secondary cardiac dilation (systolic failure) producing cardiogenic shock (hypotensionrefractory to volume resuscitation with features of end-organ hypoperfusion due to myocardial damage) and heart failure viz.chronic progressive condition where heart falls unable to pump blood enough to meet bodily demands.6-8 Additionally, MIcauses the ventricular wall or papillary muscle rupture leading to mitral regurgitation or pseudoaneurysms.9 Arrhythmias viz.change from the normal sequence of electrical impulses e.g., ventricular flutter and fibrillation and sudden cardiac death areother noted complications.10,11
Hyperglycaemia due to pre-existing, but undetected type 2 diabetes mellitus (DM), impaired glucose tolerance, orexisting insulin resistance contribute to worse outcomes post-MI, where DM is proclaimed as a group of metabolic diseasescharacterised by hyperglycaemia due to defective insulin output, action or both.12 It causes pronounced sympathetic nervoussystem activation and catecholamine secretion; predisposes to arrhythmias with QT prolongation.13 Marfella R et al. showedacute hyperglycaemia produces significant increments of QTc and QTc dispersion in normal subjects.14 Raised RBS causes pumpfailure and abolishes the response protective mechanisms viz. “ischemic preconditioning” which shields against myocardialischemic insult and ischemia-reperfusion injury.15,16 Hyperglycaemia embraces higher free fatty acid concentrations, insulinresistance and impaired myocardial glucose utilization.17,18 It induces enhanced T cell activation, both cluster of differentiation4 (CD4) and CD8 with elevated levels of C-reactive protein, interleukin-6, interleukin-18 and tumour necrosis factor (TNF)potentiating plaque instability.19,20
This delineates hyperglycaemia as a well-recognized pathogenic process for atherosclerosis and cardiovascular diseaseincluding free radical production and exhibiting lower tissue plasminogen activator activity causing increased plateletaggregation.21
This study aims to evaluate hyperglycaemic effects in patients already suffering from acute coronary syndromes (ACS)as a predictor of poor associated prognosis for it being adjoining comorbidity and increased risk of in-hospital complicationsin patients both with and without DM.
Materials and Methods
This is a hospital-based observational - analytical study conducted in Sawai Man Singh (S.M.S) Hospital, Jaipur, Rajasthan,India between June 2019 to February 2020, aimed at estimating admission RBS and HbA1c in patients of acute MI and toassess the complications arising in these patients till the time of discharge.
100 consecutive patients presenting with AMI on day 1 in departments of medicine and cardiology, between 40 to 80 yearsirrespective of sex, were taken. 50 of them with admission RBS <180 mg/mL were included in the study (group 1) and 50 withadmission, RBS >180 mg/mL were included in the study (group 2) irrespective of prior history of diabetes after applying theinclusion and exclusion criteria [the level 180 mg/mL as per the Normoglycemia in Intensive Care Evaluation Survival UsingGlucose Algorithm Regulation (NICE-SUGAR) study].22 All patients exhibiting typical rise and gradual fall of the cardiac biomarker of myocardial necrosis (cardiac troponin), confirmed using troponin T (Trop-T) (>0.017 IU/L) and creatine kinasemyocardial band (CPK-MB) (>25 IU/L) values, as found raised, with at least one of the criteria including ischemic symptoms ordevelopment of pathological Q-waves or new significant ST-segment/T-wave changes or new left bundle branch block (LBBB)in ECG; were included in the study.Furthermore, patients with comorbidities like renal or hepatic insufficiency, trauma, majorsurgery or other cardiovascular deteriorations such as aneurysms, pericardial effusion, myocarditis and valvular heart diseaseswere excluded from the study. Before participation in this study, written informed consent was obtained from all the subjects.
Serum glucose and HbA1c levels were measured along with simultaneous routine investigations like serial ECG, 2DECHO, hepatic and renal profile and delineation of systolic (SBP) and diastolic blood pressure (DBP).The patients were then followed for vitals, major events and complications like arrhythmia, heart failure, shock and deathduring the hospital stay
Statistical Analysis
All data were entered on MS Excel sheet and statistical analysis carried out using statistical software PRIMER and MedCalc.14.2.1.0. The qualitative data were expressed in proportion and percentage. The difference in proportion was analysedby using the chi-square test. The quantitative data were expressed in mean and standard deviation. Student `t’ test was usedto infer the difference in means.
Correlation analysis was done by using the Pearson’s correlation coefficient and multivariate linear regression wasperformed. Significance for tests was determined as 95% (p<0.05). Development of complications was compared betweentwo study groups and correlated with HbA1c levels.
Results
The study enrolled 100 patients who were then evaluated based on multiple factors viz., age, sex, body mass index (BMI),smoking status, h/o DM and hypertension, which were then compared for significance.
Table 1 shows the mean age in group 2 was slightly higher (62.26 years) as compared to group 1 (62.22 years, but thedifference was not statistically significant (p>0.05).
Table 1. Comparing the demographic profile and risk factors of the study groups. Abbreviations: DOF- Degrees offreedom; MV- Multivariate; NS- Non-significant; S- Significant; SBP- Systolic blood pressure; DBP- Diastolic bloodpressure. A p-value of less than 0.05 was considered statistically significant.
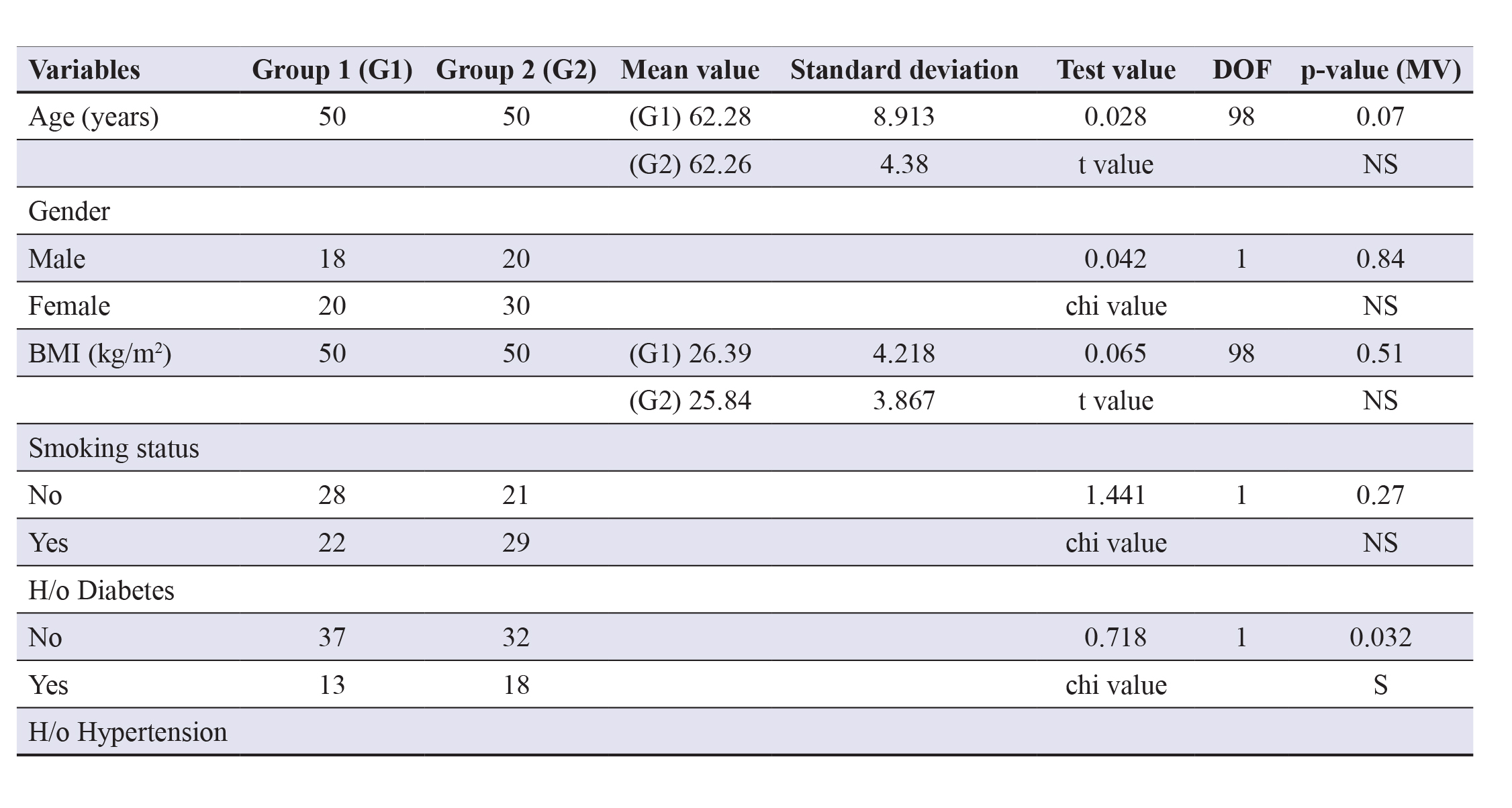
Gender wise group 1 had more males (64%) as compared to group 2 (60%) and the mean BMI in group 1 (26.39 kg/m2)is slightly higher in contrasting group 2 (25.84 kg/m2), but the differences were statistically insignificant (p>0.05).
Table 1 defines the risk factors including smoking status showing that 29 (58%) subjects in group 2 were smokers,whereas only 22 (44%) in group 1 were smokers. H/o diabetes was more in group 2 (36%) contrasting group 1 (26%) and h/ohypertension (BP >140/90) was higher (56%) in group 2 as compared to group 1 (40%) when taken for multivariate analysisproved statistically significant (p<0.05), establishing them as independent risk factors for complicating MI prognosis.
The mean heart rate was comparatively higher in group 2 (111.8/min) contrasting group 1 (84.94/min) which wasstatistically significant (p=0.001). The mean SBP was higher in group 1 (126.4 mmHg) vs. group 2 (108.6 mmHg). Further,the mean DBP was more in group 1 (89.44 mmHg) contrasting group 2 (75.24 mmHg) and both were found to be statisticallysignificant (p<0.05).
Also, the mean respiratory rate in group 2 (22.04/min) was higher than group 1 (17.48/min) which too was statisticallysignificant (p<0.001).
Overall, these observations revealed that the two groups were comparable taking into account all the confounding factors.There were pre-existing hypertension and concurrent diabetes in maximum patients of group 2 respectively that predisposedthem to subsequently cause comorbidities and as expected, suggested them to independently deteriorate the patient viability.
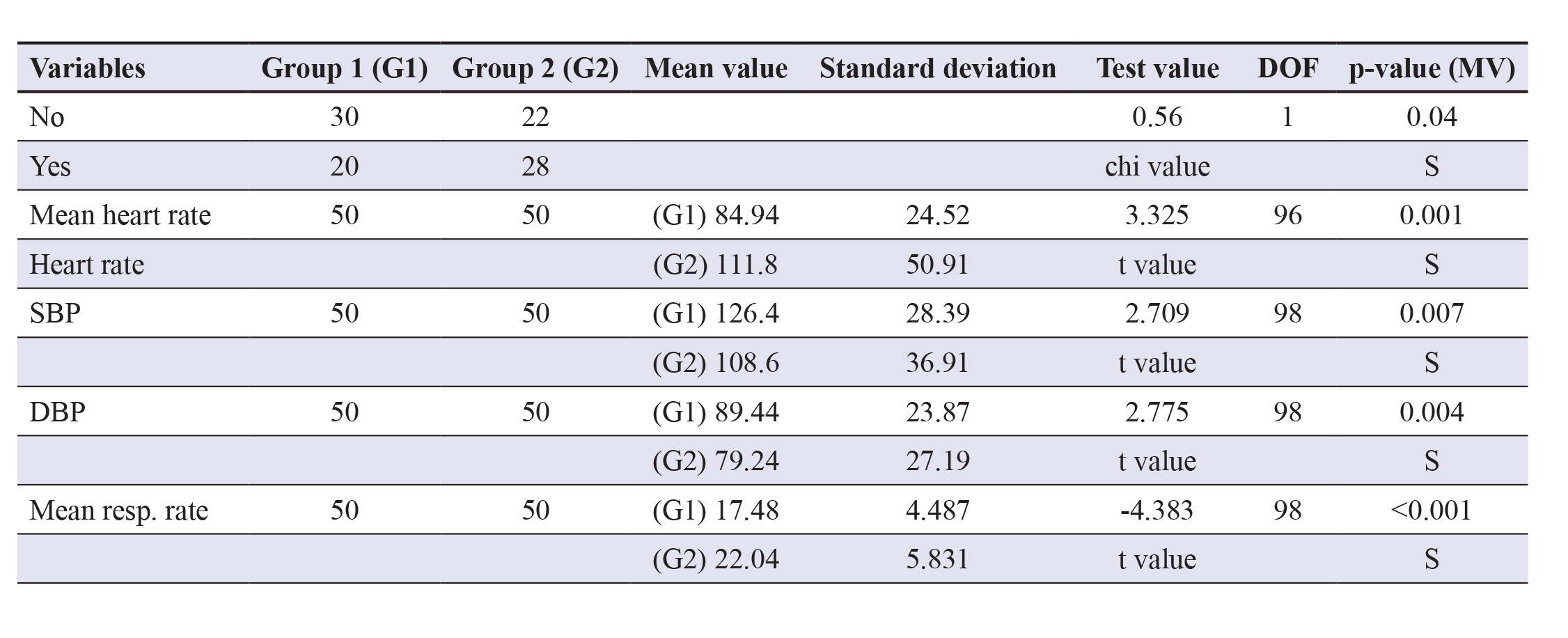
Table 2 delineates that adversities like basal crepitations were observed more frequently in group 2 (36%) as comparedto group 1 (8%). Presence of S3 gallop was found in 34% of group 2 subjects and 6% of group 1 subjects where boththese differences were statistically significant (p<0.05). Bilateral pedal oedema occurred more in group 2 subjects (8%) ascompared to group 1 subjects (4%) but was statistically insignificant (p>0.05). Furthermore, heart failure as a complicationwas seen in 36% group 2 subjects and only 8% group 1 subjects which were statistically significant (p<0.05).
Multiple arrhythmias and cardiogenic shock were reported in 30% group 2 subjects each, detected on cardiac monitoring,contrasting only 6% cases in group 1 and the difference was statistically significant (p=0.004).
Table 3 signifies HbA1c and its statistically significant differences in the complication of AMI (p<0.001). It revealsthat death as an outcome occurred in 17.3% group 2 subjects and 16.7% group 1 subjects but revealed to be statisticallyinsignificant (p>0.05). In-hospital management was given as per the American Heart Association (AHA) guidelines includingdrugs viz., metoprolol and statins viz., atorvastatin usage for patient recovery.
Low dose aspirin was given both prophylactically and therapeutically and all feasible revascularizations were carried outwhile simultaneous response on improvement and complications was monitored and documented.
Table 2. Comparison and analysis of the complications of AMI. Abbreviations: DOF- Degrees of freedom;NS- Non-significant; S Significant.
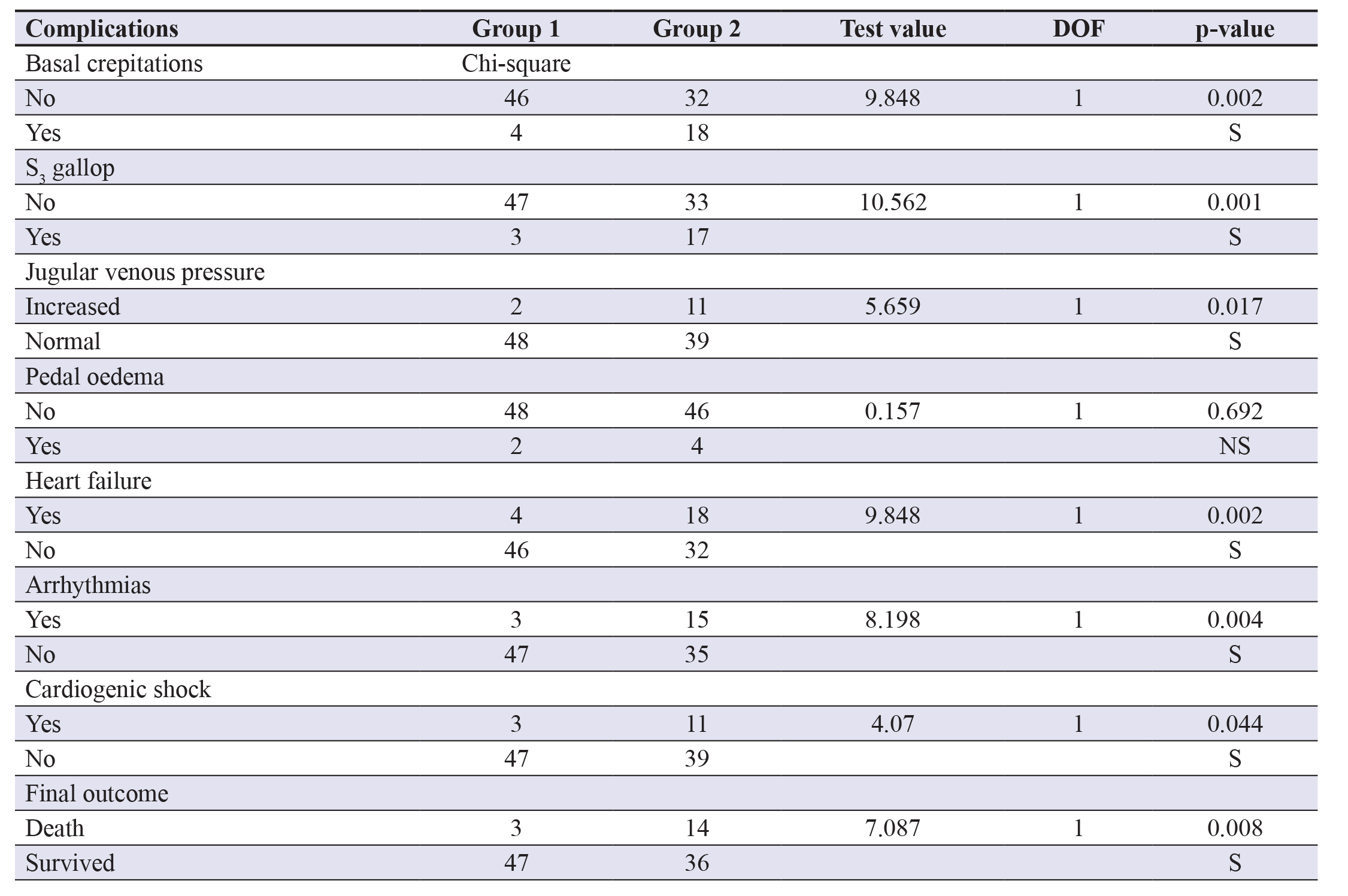
Table 3. Prognostic value of HbA1c on variable outcomes. Abbreviations: HbA1c- Haemoglobin A1c; RBS- Randomblood sugar; DOF- Degrees of freedom; NS- Non-significant; S- Significant.
Discussion
In our study regarding the association of admission RBS and HbA1c with complications in the patients of acute MI, it wasobserved that complications such as heart failure, shock, arrhythmias and demise did occur. Mainly, failure was present in 8%of group 1 patients while it was 36% in group 2 patients. This was significant (p=0.002), indicating incidence of heart failureis more in the group with higher RBS, where known diabetics formed a major proportion
Clinical findings like basal crepitations, S3 gallop, jugular venous pressure (JVP) and pedal oedema were also compared,which suggested a significant difference highlighting the higher incidence of heart failure in group 2. It was recorded thatarrhythmias and cardiogenic shock occurred more frequently with higher blood sugar as in group 2. Mortality was only 6%in group 1, whereas was 28% in group 2 patients with a p-value of 0.008, indicating highly significant difference attributedto hyperglycaemia.
Prognostic role of raised RBS in AMI has been validated by many studies e.g., Milvidate et al. who studied the frequencyof admission hyperglycaemia and abnormal glucose tolerance at discharge in patients with acute MI and no previous h/oDM and the results proved in concordance to our results.23 Mansour et al. studied regarding hyperglycaemia on arrival forpatients presenting with ACS, irrespective of diabetic status and concluded using logistic regression with heart failure as thedependent variable attributed to admission acute phase hyperglycaemia (OR=2.1344, 95% CI=1.0282-4.4307; p=0.0419)comparable to ours where AMI led to 30% of cases of heart failure.24
Higher mortality rates viz. 28% in hyperglycaemic patients were recorded in our study; similar to Meier et al. whichshowed higher long-term mortality rates and larger infarct size (measured by creatine kinase and MB fraction levels) among116 hyperglycaemic AMI patients with 18% mortality.25
Interestingly, Oswald and Smith et al. in their two studies assessed the prevalence of undiagnosed DM (using HbA1c)in patients admitted with AMI and the effect of DM on outcomes and reported 33% mortality (>7.5%) and 63% (>8.5%)whereas it was 18% in our observations correlated with HbA1c >6%.26
In our study, multiple confounding risk factors showed statistically no significant differences between the groups depictingthe comparable composition of the groups.
Zaghlaa et al. assessed the impact of admission RBS on the hospital course and outcome in patients presenting withAMI in the intensive care unit (ICU) with 50 cases and elaborated that there was a significant correlation between RBS andh/o DM and h/o smoking (p=0.000 and 0.008, respectively) and also between RBS and complications of MI including sinustachycardia, arrhythmia, and ICU length of stay (p=0.008, 0.002, and 0.000, respectively); which was statistically significanton multivariate regression, even in our study (p<0.05 each, respectively). In the HbA1c prognostic scenario, 12.5% patientsdeveloped arrhythmia in group 1 and 23.1% patients in group 2, whereas cardiogenic shock was present in 10.44% patientsin group 1 while 15.45% of patients in group 2 and both differences were insignificant (p>0.05).27
Lazzeri et al. assessed the prognostic role of HbA1c for mortality at short and long terms in 518 consecutive STEMIpatients without h/o DM and observed that higher HbA1c values pinpoint a subset of patients who, in the early phase ofSTEMI, show an abnormal glucose response to stress indicated by dysglyceamia, worse glycaemic control during intensivecardiac care unit (ICCU) stay (peak glycaemia) and a higher incidence of acute insulin resistance (homeostatic modelassessment index) in comparison to the evident complications of AMI that raised glycated Hb predisposed to.28
The comparative analysis in our study thereby showed that elevated RBS in patients with AMI appears more importantthan prior long-term abnormal glucose metabolism (detected by elevated HbA1c) in predicting outcome in patients withAMI and this may be because of a stress response accompanied by high levels of catecholamines and cortisol which increaseglycogenolysis and lipolysis and reduce insulin sensitivity, resulting in elevated glucose levels.
Therefore, hyperglycaemic patients due attributed to that response; cause more severe haemodynamic compromise ormore extensive myocardial damage thereby worsening the prognosis.
Limitation of the Study
Our study has a limitation of one-time glucose estimation irrespective of timing and previous meals which may vary innormal individual also.
Conclusion
We know that IHD is a common cause of death worldwide and is likely to become the most common cause of death globallyby 2020. Our comparative analysis showed elevated glucose or stress hyperglycaemia common in AMI patients and RBS asan important predictor of adverse outcomes.
This observational study aimed at highlighting the importance of measuring serum glucose levels as a viable indicatorof further morbidities in an already morbid state of MI using advanced modalities viz., spectrophotometry for glucoseand immune-turbidimetric tests for HbA1c levels and highlights the clear-cut difference in occurrence and incidence ofcardiovascular complications in hyperglycaemic patients suffering from MI thus establishing a positive association betweenthe two factors.
Declaration of conflicting interest
The author declares the absence of any conflict of interest.
Funding
No cost was born by any participant and no grant or funding was sought as all the performed investigations are done free ofcost as per the Government of Rajasthan policies.
Ethical approval
Since the study is an observational study, there is no need for any ethical committee approval.
References
1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics—2019Update: A Report From the American Heart Association. Circulation. 2019;139:e56-e528.
2. Thygesen K, Alpert JS, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction.Universal Definition of Myocardial Infarction. Eur Heart J. 2007; 28:2525-2538.
3. Kristian T, Allan S, Chaitman BR, Bax JJ, Morrow DA, White HD, et al. European Society of Cardiology. Universal definitionof Myocardial Infarction. Circulation. 2018; 138(20):e618-e651.
4. Cotran RS, Kumar V, Robbins SL (eds). Robbins Pathologic Basis of Disease. 5th ed. Philadelphia: WB Saunders; 1994.
5. Rubin E, Farber JL. eds. Essential textbook of pathology. 2nd ed. Philadelphia: JB Lippincott, 1995; reprinted 2016
6. Massaro AF. Approach to the Patient with Shock. In: James JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds.Harrison’s Principles of Internal Medicine. 20th ed. New York, NY; 2018:Ch296. Available at www.harrisonsim.com.
7. Vahdatpour C, Collins D, Goldberg S. Cardiogenic Shock. J Am Heart Assoc. 2019; 8(8):e011991.
8. Vedin O, Lam CSP, Koh AS, Benson L, Teng THK, Tay WT, et al. Significance of ischemic heart disease in patients with heartfailure and preserved, midrange and reduced ejection fraction: a nationwide cohort study. Circ Heart Fail. 2017; 10(6):e003875.
9. Davis N, Sistino JJ. Review of Ventricular Rupture: Key Concepts and Diagnostic Tools for Success. Perfusion. 2002; 17(1):63-7.
10. John RM, Stevenson WG.Approach to Ventricular Arrhythmias.In: James JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. 20th ed. New York, NY; 2018:Ch247. Available at www.harrisonsim.com
11. Luqman N, Sung RJ, Wang CL, Kuo CT. Myocardial ischemia and ventricular fibrillation: pathophysiology and clinical implications. Int J Cardiol. 2007; 119(3):283-290.
12. Powers AC, Niswender KD, Evans-Molina C. In: James JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds.Harrison’s Principles of Internal Medicine. 20th ed. New York, NY; 2018:Ch396. Available at www.harrisonsim.com.
13. Koraćević G, Vasiljević S, Velicković-Radovanović R, Sakac D, Obradović S, Damjanović M, et al. Stress Hyperglycemia inAcute Myocardial Infarction. Vojnosanit Pregl. 2014; 71(9):858-69.
14. Marfella R, Nap F, De Angelis L, Siniscalchi M, Rossi F, Giugliano D. The effect of acute hyperglycaemia on QTc duration inhealthy man. Diabetologia. 2000; 63(5):571-75.
15. Iwakura K, Ito H, Ikushima M, Kawano S, Okamura A, Asano K, et al. Association between hyperglycemia and no reflowphenomenon: a study. J Am Coll Cardiol. 2003; 41:1-7.
16. Kersten JR, Schmeling TJ, Orth KG, Pagel PS, Warltier DC. Acute hyperglycemia abolishes ischemic preconditioning in vivo.Am J Physiol. 1998; 275:H721-H725.
17. Tansey MJ, Opie LH. Relation between plasma free fatty acids and arrhythmias within the first twelve hours of acute MI. Lancet. 1983; 2:419-422.
18. Oliver MF. Metabolic causes and prevention of ventricular fibrillation during acute coronary syndromes. Am J Med. 2002;112:305-311.
19. Morohoshi M, Fujisawa K, Uchimura I, Numano F. Glucose-dependent interleukin 6 and tumor necrosis factor production byhuman peripheral blood monocytes in vitro. Diabetes. 1996; 45:954-959.
20. Esposito K, Nappo F, Marfella R, Giugliano G, Ciotola M, Quagliaro L, et al. Inflammatory cytokine concentration are acutelyincreased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002; 106:2067-2072.
21. Pandolfi A, Giaccari A, Cilli C, Alberta MM, De Filippis EA, Buongiorno A, et al. Acute hyperglycemia and acute hyperinsulinemia decrease plasma fibrinolytic activity and increase plasminogen activator inhibitor type 1 in rat. Acta Diabetol. 2001;38:71-76
22. NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Yu-Shuo Su S, Blair D, Foster D, Dhingra V, et al. Intensive VersusConventional Glucose Control in Critically Ill Patients. N Engl J Med. 2009; 360(13):1283-297.
23. Milvidaite I, Slapikas R, Statkeviciene A, Babarskiene MR, Luksiene D, Slapikiene B. Admission hyperglycemia and abnormalGTT at discharge in patients with acute myocardial infarction and no history of diabetes mellitus. Medicina (Kaunas). 2007;43(12):935-41
24. Mansour AA, Wanoose HL. Acute Phase Hyperglycemia among Patients Hospitalized with Acute Coronary Syndrome: Prevalence and Prognostic Significance. Oman Med J. 2011; 26(2):85-90.
25. Meier JJ, Deifuss S, Klamann A, Launhardt V, Schmiegel WH, Nauck MA. Plasma glucose at hospital admission and previousmetabolic control determine myocardial infarct size and survival in patients with and without type 2 diabetes: the LangendreerMyocardial Infarction and Blood Glucose in Diabetic Patients Assessment (LAMBDA). Diabetes Care. 2005; 28:2551-2553.
26. Oswald GA, Corcoran S, Yudkin JS. Prevalence and risks of hyperglycaemia and undiagnosed diabetes in patients with acutemyocardial infarction. Lancet. 1984; 1:1264-1267.
27. Zaghla HE, Elbadryb MA, Ashour AA, Abdelfata MM. Influence of admission blood glucose and hemoglobin A1c on outcomeof acute MI. Egypt J Intern Med. 2014; 26:21-6.
28. Lazzeri C, Valente S, Chiostri M, D’Alfonso M, Gensini GF, Spini V, et al. Admission glycaemia and acute insulin resistancein heart failure complicating acute coronary syndrome. Heart Lung Circ. 2015; 24(11):1074-080.
